Moderna uses an efficient method to make large quantities of individual mRNAs with high purity, based on a technique developed in 1984 by D. Melton and colleagues at Harvard University and published in Nucleic Acids Research. In here bacteriophage polymerases were used to transcribe plasmid DNA and make purified RNAs. D. Melton went further and showed that he could transcribe a synthetic mRNA injected into frog oocytes, and get expression of b-globin (Krieg & Melton, Nucleic Acids Research, 1984). With this report, the protocol to make pure mRNAs was settled.
Today, at Moderna, they moved on from this idea to a GMP drug-product inside of a vial in under two months. In the case of the COVID-19 vaccine, they went from a sequence of the virus to having the material ready for a Phase I clinical trial in 45 days.
This automated process starts with back-translation using Moderna’s proprietary computer algorithm, that has been defined over the years to pick the best mRNA sequence for the target in question.
Then, the therapeutic mRNA is engineered to avoid the innate immune sensors that defend against RNA viruses and might destroy the vaccine. These defences come in to main types: Toll-like receptors (TLR) in the endoplasmic reticulum, and RIG-I/MDA-5-like receptors in the cytoplasm.
To avoid TLRs, Moderna places modified nucleotides. This technique is based on a paper by Diebold and colleagues published in Science (2004), where the researchers took macrophages from mice and incubated them with different polymers (polyA, polyC, polyG, polyI, polyU), then checking for an IFN-alpha response. What they have seen was that the only polymer that created the IFN-alpha response was the one that contain Uridine (polyU), which seems to be the only nucleotide that is being recognized by the TLR-driven innate immune response. As such, at Moderna, mRNAs are engineered to replace all U’s with N1-methyl-pseudiuridine without affecting base-pairing, but avoiding TLR recognition. N1-methyl-pseudiuridine is a naturally occurring nucleotide that our bodies recognize as native.
Another problem though, is avoiding the creation of double-stranded RNA (dsRNA) to escape the RIG-I/MDA-5-like receptors. The state-of-the-art used in Moderna to avoid this trap, is HPLC purification to reduce dsRNA content. This is a technique based on Karikó & Weismann (2011, Nucleic Acids Research), that results in very little dsRNA in the end-product, and an avoidance of the innate immune response. The developed technique at Moderna has recently been published in Science Advances (June 2020), where Moderna scientists describe a highly sensitive assay to detect dsRNA and purify therapeutic mRNAs.
(It seems Moderna scientists are now going even further, and have reengineer T7 RNA polymerase not to produce dsRNA at all – although this has not been published yet)
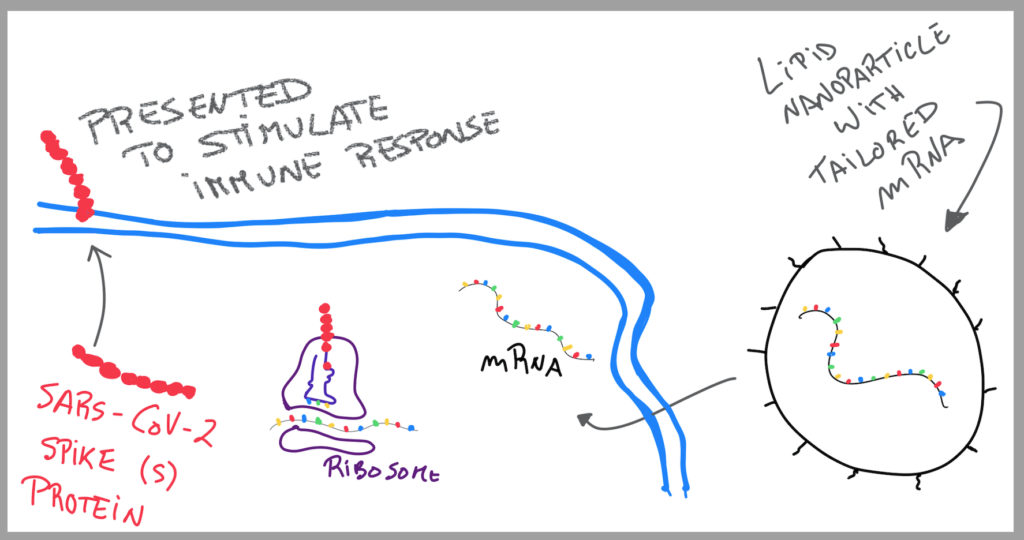
The third step to make a good mRNA-drug, and a successful vaccine, is to find an efficient delivery method. With that in mind, Moderna researchers looked back at work done by Wolff and colleagues in the 90s (Science, 1990, vol.247, 1465-1468), where they were able to directly inject RNA in the mouse skeletal mouse and have protein expression, with no special delivery system involved.
In 2014, researchers at Moderna were able to go further, and measured with a Luciferase system, the in vivo expression of biologically active proteins in a dose-dependent manner, by injecting mice with Moderna’s naked mRNA. Nowadays, Moderna targets different tissues via multiple Routes of Administration with specific delivery vehicles (ROAs: direct injection in the heart or tumours, subcutaneous, intravenous, intramuscular).
Most ROAs require mRNA encapsulation with Lipid nanoparticles (LNPs, 80-100nm). Each encased particle contains 2-6 molecules of mRNA, phospholipid cholesterol, a PEG lipid that keeps the lipid nanoparticles stable avoiding aggregation, and an ionizable lipid that at low pH interacts with the mRNA. These mRNA-LNPs are akin to endogenous lipid transport complexes, slightly bigger than Very-Low Density Lipoproteins (VLDLs), and seen as “friendly bubbly characters” by our bodies.
At Moderna, they empower a rational-structure LNP design by analysing and engineering each individual step of the component mixture, to enhance chemical/physical stability of mRNA-LNPs to allow an optimal biodistribution, cellular uptake, endosomal escape and protein expression. In comparison to the competitor LNPs in the field, which have a 1-2% delivery rate, Moderna’s LNPs can deliver 30% mRNA into cells. To date, Moderna has published two peer-revied articles describing these efforts and their applications in different ROAs (Sabis … Benenato, Mol Ther, 2018; Hassett … Britto, Mol Ther Nuc Acids, 2019).
Finally, there needs to be the right knowledge to engineer the best mRNA sequence for a particular purpose in order to make a good mRNA-drug or vaccine.
So, there needs to be translation initiation fidelity, so that it always starts in the right place and there’s faithful decoding; and, also, the ribosome needs to stop in the right place.
The mRNA needs to have a functional half-life, to function before it gets destroyed.
Also, the therapeutics or vaccine needs to hit the correct cell type, which is achieved by putting “off-logic gates”, like microRNAs target sites in the 3’prime UTR, that cause the mRNA to be degraded in case it hits an undesired cell type.
At Moderna, they have all steps in place for a rapid development of mRNA as medicines:
- Efficient methods to make large quantities of individual mRNAs at high purity;
- Ability to avoid the innate immune sensors that defend against RNA viruses;
- Efficient delivery methods,
- Knowledge of how to engineer the best mRNA sequence for a particular purpose.
Besides proprietary algorithm to define the mRNA sequence and unique LNP design; one of the big differences between BioNTech/Pfizer vaccine and Moderna, is that Moderna does not use self-amplifying RNA in their vaccines. According to Dr Melissa Moore, Chief Scientific Officer at Moderna Therapeutics Inc., self-amplifying RNA first starts by building dsRNA which pushes-up the immune response against the vaccine, as such Moderna hasn’t gone down that road to avoid such road-blocks.
The important take-out message is not which vaccine we take.
With a mRNA vaccine, the cell is doing the protein itself, and the antigen-presenting cells are exhibiting it on their surface, which causes an activation of B and T cells.
This protects us and others from developing this devastating disease.