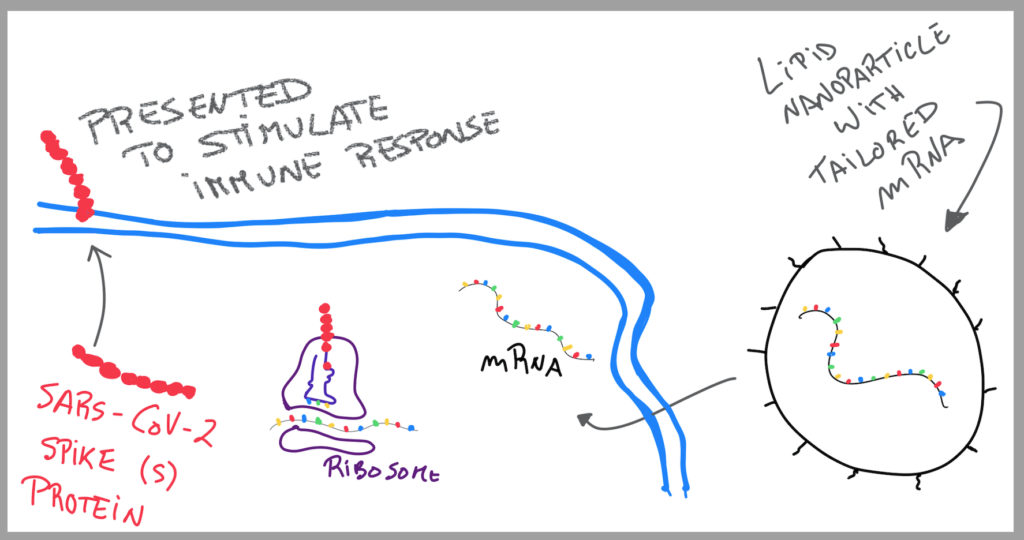
The principle of mRNA therapeutics is to introduce therapeutic messenger (m) RNAs encoding the genetic information for a protein, into a cell of interest. The mRNA structure is designed to increase half-life, translation and protein functionality.
The mRNA synthesis is made by in vitro transcription using a linear DNA template, which provides more than 500 copies of mRNA per template. They promote a transient expression of the encoded protein/antigen, and are degraded into nucleotides, without the formation of toxic metabolites. Since mRNA is highly sensitive, it gets degraded after a short-time, with no risk of genomic integration.
This one process can be used to manufacture essentially any mRNA sequence.
The raw reaction mixture has mRNA and all types of impurities, both process-related (T7 RNA polymerase, remaining building blocks, hydrolyzed DNA…), and product-related impurities (break-off transcripts, side products…), that need to be further purified with high affinity chromatographic methods.
Once purified RNA is obtained, the therapeutic is ready to deliver to the patient.
BioNTech together with their partner Pfizer and FosunPharma, managed to do all this in a record time of 84 days to develop a COVID-19 vaccine.
Their scientists designed multiple antigen variants using the SARS-CoV-2 Spike (S) protein.
Next, they ran preclinical and toxicology evaluation tests in vitro and in vivo (e.g., in vitro expression data, antibody titers in animal models, pseudo-virus neutralization (pVN) assay in animal models), so they could discover the most active antigen and bring it into the clinic. Also, using their Good Manufacturing Practice (GMP) facilities they produced small scale batches for fast entry into the clinic to demonstrate safety, tolerability (i.e., low reactogenicity), and immunogenicity.
Afterwards, they initiated clinical trials, where not one but four vaccine candidates were simultaneously studied, in order identify the safest and most effective candidate for further development. In parallel, there was already preparation of larger manufacturing batches of the candidate with the best output in the clinical studies; and, the identification of collaboration partners to develop and provide the vaccine worldwide (Pfizer, FosunPharma).
BioNTech and Pfizer studied three mRNA types during all Phase I/II studies, to evaluate safety and efficacy. The basis for such studies was that different types of mRNA vaccines lead to different responses.
As such, vaccine prototype (1) was a Uridine mRNA (uRNA) with strong intrinsic adjuvant effect, strong antibody response, that stimulates the production of more CD8+T cells then CD4+T cells.
Vaccine prototype (2) was a Nucleoside-modified mRNA (modRNA), with a moderate intrinsic adjuvant effect, very strong antibody response, lower cytokine induction, that stimulates more CD4+T cells then CD8+T cells.
In the case of vaccine prototype (3), this was a Self-amplifying mRNA (saRNA) with long duration, and a higher likelihood for good efficacy antibody response with lower dosage.
Early and constant interaction with the regulatory agencies (e.g., EMA) has allowed that BNT162b2 vaccine candidate raced to the finish line, being now the leading candidate from BioNTech & Pfizer; and, currently in review for conditional marketing authorization approval.
It is outstanding that if a new variant of the virus is required (e.g., due to a mutated virus sequence), a new GMP batch of a possible vaccine could be available within half of the time (around 42 days).
If there is a positive message out of these dark days, is that Science is there to save us from our doom.
Let’s embrace Science.